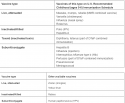
Because it most resembles the original virus with its motifs in native conformations.
The other one is live attenuated vaccine which is live viruses modified to be less virulent and dangerous. However, in some cases, they mutate and become dangerous again. Experts avoid this method nowadays.
All the hype on mRNA or Adenovirus vaccines is just hype. They're all startups whose executives want to dump stocks.
The big players like Merck are all working on inactivated or subunit vaccines. The latter of which is more difficult but safer as it only uses a small portion of the virus.
An inactivated vaccine is the quickest way to get to a working vaccine, usually.
Localizer, thanks for the explanation.
If what you said is true, then China is way way ahead in Covid-19 vaccine development because all the leading vaccines are :
- CNBG (China / 2 vaccines) and Sinovac (China) are inactivated vaccine in human clinical trial.
- Moderna (US), Imperial College (UK), BioNTech (Germany) are all mRNA vaccines
- CanSino Biologic (China) and Oxford University (UK) are Adenovirus vaccines
- No other inactivated vaccines worldwide have started human clinical trial