That's exactly the point. Thank you for agreeing with me that
unequal distribution of age composition exists. "
Quite a few" = unequality = systematic bias in interpretation of results.
Since they are not equal in two-dose vaccination (or even one-dose vaccination), there is a systematic bias in the interpretation in the results due to unequal age distribution. "Quite a few" is not equal, you need equality in age distributions to avoid systematic bias, "Quite a few" is not sufficient.
Also, according official UK data on vaccination rates by age bands.
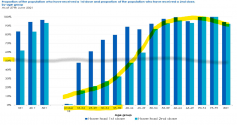
Younger people are significantly less likely to be two-dosed vaccinated compared to older people.
If you have unequal distribution of age in the vaccinated or unvaccinated cohort (two-doses), then you have a systematic bias in the results.
View attachment 74340
Source:
UK only started offering vaccines to <40 age people starting in
, so how can young people (dead last in priority) be more likely to be vaccinated when they weren't even offered the vaccine until just recently? Old people have been receiving vaccines since April, almost 3 months earlier.
Also, the official UK data chart shows younger people are less likely to be vaccinated, based on how phase 2C of vaccination is rolled out. The data clearly shows younger are less vaccinated.
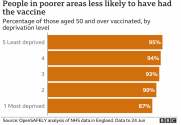
Indeed, richer people are more likely to be vaccinated, see chart (95% vs 87%).
View attachment 74341
and rich people have better health outcomes, with or without vaccines.
UK is a single-payer healthcare system with universal healthcare. Even the poorest people have healthcare insurance, and therefore access to healthcare. UK NHS is not limited only to the rich folks. Everyone has access to healthcare insurance, regardless of education status either.
So Age and Comorbidity are likely the biggest predictors.
Then why do you value low-quality self-reported observational data such as active surveillance database that isn't even intended to establish causality, only correlation?
What are these different factors, I am curious....
The "results" you are citing are self-reported case series data, the lowest quality of evidence in the hierarchy of level of evidence. They are very susceptible to systematic bias if you are not controlling for confounding factors. That's why scientists don't take this data seriously, only to provide association/correlation, but NEVER to prove causality due to so many risks and biases inherent in the study design.
There is, it's called the Pfizer/Moderna RCT Trials. The data already exists, it adjusts for confounding using randomization. You are actively seeking sources of data that have high likelihood of bias due to lack of randomization.
There is no confounding factors due to randomization. There are confounding variables in your self-reported case series observational due to LACK of randomization.
Randomization in Pfizer/Moderna/AZ/Sinopharm/Sinovac trials eliminate or minimize all confounding variables, so they aren't an issue.
You clearly don't have any clue about epidemiology, biostatistics, or clinical trial designs. I advise you to look up how to control for confounding variables in RCTs and observational study designs.
From what I understand, Sinopharm's clinical trial protocol used a too stringent inclusion criteria for non-COVID cases, so it reduced efficacy number for Sinopharm. It has nothing to do with confounding factors, but how cases and non-cases were identified in the inclusion critieria. You are mixing up confounding variables with the inclusion criteria and patient identification within a study protocol.